Pre lab for build an atom – Embark on an atomic adventure with our pre-lab for building an atom! In this engaging exploration, we’ll delve into the fundamental structure of matter, uncovering the secrets of protons, neutrons, and electrons. Prepare to construct your own atomic model and unravel the mysteries of the periodic table.
Get ready to visualize atomic structures using innovative techniques and discover the practical applications of atomic knowledge in various scientific fields. Join us on this captivating journey into the heart of matter!
Pre-Lab Preparation
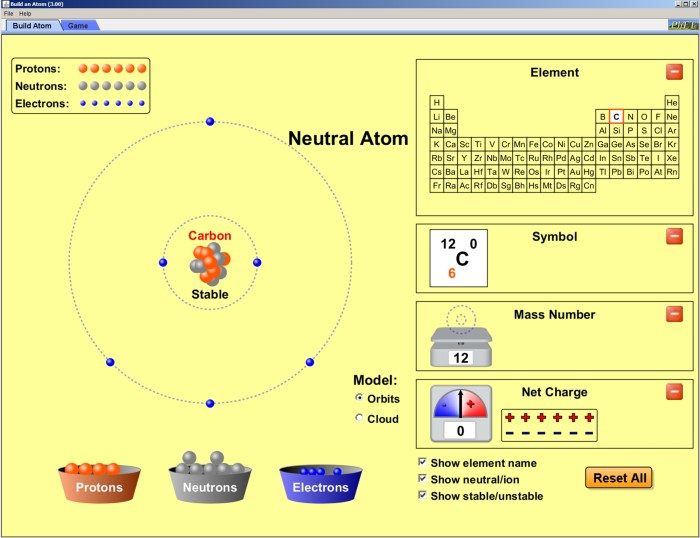
The pre-lab is an essential component of the lab experience that prepares you for the hands-on work to follow. It provides an overview of the experiment, including its purpose, objectives, and safety precautions. By completing the pre-lab, you will gain a better understanding of the experiment and be able to participate more effectively in the lab session.
Pre-lab for “Build an Atom” is a fun way to learn about the structure of atoms. The lab requires students to use a variety of materials, including marshmallows, toothpicks, and pipe cleaners. By following the instructions in the pre-lab, students will be able to create a model of an atom that accurately represents the number of protons, neutrons, and electrons in the atom.
You might even want to check out legal alien by pat mora for more inspiration. Once students have completed the pre-lab, they will be ready to begin the lab and build their own atom models.
Required Materials and Equipment
- Lab coat
- Safety goggles
- Gloves
- Calculator
- Periodic table
- Model building materials (e.g., pipe cleaners, beads, straws)
Safety Precautions and Proper Lab Etiquette, Pre lab for build an atom
- Always wear appropriate personal protective equipment (PPE) in the lab, including a lab coat, safety goggles, and gloves.
- Follow all instructions carefully and do not deviate from the experimental procedure.
- Be aware of your surroundings and be mindful of others working in the lab.
- Keep your work area clean and organized.
- Dispose of all waste materials properly.
- Report any accidents or spills to the instructor immediately.
Introduction to Atomic Structure
In the realm of chemistry, understanding the structure of matter is crucial. The fundamental building blocks of all substances are atoms, microscopic entities that govern the chemical and physical properties of materials.
An atom consists of three fundamental subatomic particles: protons, neutrons, and electrons. Protons and neutrons reside in the atom’s nucleus, while electrons occupy the space surrounding the nucleus.
Atomic Number and Mass Number
Each element is uniquely identified by its atomic number, which represents the number of protons in its nucleus. The mass number, on the other hand, is the sum of protons and neutrons present in the nucleus.
Isotopes
Atoms of the same element can have varying numbers of neutrons, giving rise to isotopes. Isotopes share the same atomic number but differ in mass number. They exhibit similar chemical properties but may have distinct physical characteristics.
Building an Atom Model
Building an atom model is an excellent way to visualize and understand the structure of atoms. This activity requires simple materials and provides a hands-on approach to learning about the fundamental particles that make up matter.
Materials Required
- Styrofoam balls (different sizes)
- Pipe cleaners
- Toothpicks
- Markers or paint
Steps Involved
- Determine the number of protons, neutrons, and electrons:Use the periodic table to find the atomic number (number of protons) and atomic mass (number of protons + neutrons) of the element you want to model.
- Create the nucleus:Use a large Styrofoam ball to represent the nucleus. Mark the number of protons on the ball with a marker or paint.
- Add neutrons:Use smaller Styrofoam balls to represent neutrons. Add the number of neutrons to the nucleus.
- Create electron shells:Use pipe cleaners or toothpicks to create electron shells around the nucleus. The number of shells depends on the energy levels of the electrons.
- Add electrons:Use small Styrofoam balls or beads to represent electrons. Attach them to the electron shells.
Tips
- Use different colors for protons, neutrons, and electrons to make the model easier to visualize.
- Label the different parts of the atom to reinforce understanding.
- Use a scale to ensure the model is proportionate.
Representing Atomic Structure: Pre Lab For Build An Atom
To represent the atomic structure of an element, we use chemical symbols, which are one or two letters that uniquely identify each element. The periodic table is a tabular arrangement of elements organized by their atomic number, electron configuration, and recurring chemical properties.
It provides a systematic way to identify and compare elements.
Electron Configuration
The electron configuration of an element describes the arrangement of its electrons in its atomic orbitals. It is written as a string of numbers and letters that indicate the energy levels, subshells, and number of electrons in each.
Visualizing Atomic Structure

Visualizing atomic structure is a crucial step in understanding the behavior and properties of elements. Different visualization techniques offer unique perspectives on the arrangement of electrons and nuclei within an atom, providing valuable insights for chemists and physicists.
Bohr Model
The Bohr model, proposed by Niels Bohr in 1913, depicts the atom as a miniature solar system, with electrons orbiting the nucleus in discrete, circular paths. This model successfully explained the emission and absorption spectra of hydrogen atoms, providing the foundation for understanding atomic energy levels.
Advantages:
- Simple and intuitive, providing a clear mental image of atomic structure.
- Explains the quantization of electron energy levels and the emission and absorption of light.
Limitations:
- Does not account for the wave-particle duality of electrons.
- Cannot explain the behavior of atoms with more than one electron.
Lewis Dot Structures
Lewis dot structures, developed by Gilbert N. Lewis in 1916, represent atoms as symbols surrounded by dots representing valence electrons. These structures emphasize the bonding behavior of atoms, showing how they share or transfer electrons to achieve a stable configuration.
Advantages:
- Simple and easy to draw, providing a quick visual representation of atomic bonding.
- Predicts the geometry and stability of molecules, helping to understand their chemical properties.
Limitations:
- Does not provide information about electron energy levels or the shape of orbitals.
- Cannot represent all types of chemical bonding, such as covalent bonds with multiple bonds or resonance structures.
Comparison of Visualization Techniques
The following table summarizes the key differences between the Bohr model and Lewis dot structures:
| Characteristic | Bohr Model | Lewis Dot Structures |
|---|---|---|
| Focus | Electron energy levels and atomic spectra | Chemical bonding and molecular geometry |
| Representation | Circular orbits of electrons | Symbols with dots representing valence electrons |
| Advantages | Simple and intuitive, explains quantization | Simple and predictive for bonding behavior |
| Limitations | Does not account for wave-particle duality, multi-electron atoms | Does not provide information on electron energy levels, orbital shapes |
Applications of Atomic Structure
Understanding atomic structure has profound implications in various scientific fields. It enables scientists to predict the chemical properties and reactivity of elements, paving the way for advancements in chemistry, physics, and biology.
- Chemistry:Atomic structure helps predict chemical bonding and the formation of molecules. This knowledge is crucial for designing new materials, pharmaceuticals, and industrial processes.
- Physics:Understanding atomic structure aids in comprehending the behavior of electrons in materials, leading to the development of semiconductors, transistors, and other electronic devices.
- Biology:Atomic structure plays a vital role in understanding the interactions between biological molecules, such as proteins and DNA, and their functions within living organisms.
Real-world applications of atomic structure include:
- Nuclear energy:Atomic structure principles are applied in nuclear power plants to generate electricity.
- Medical imaging:X-rays and magnetic resonance imaging (MRI) rely on atomic structure to provide detailed images of the human body for diagnostic purposes.
- Nanotechnology:Atomic structure manipulation enables the creation of advanced materials with unique properties for use in electronics, optics, and biomedical applications.
Commonly Asked Questions
What is the purpose of a pre-lab?
A pre-lab helps you prepare for a lab experiment by providing essential information, safety guidelines, and step-by-step instructions.
Why is it important to understand atomic structure?
Understanding atomic structure is crucial for comprehending the properties and behavior of elements, as well as their interactions in chemical reactions.
What are some practical applications of atomic structure?
Atomic structure knowledge finds applications in diverse fields, including chemistry, physics, biology, and materials science.