Rank the indicated protons in order of increasing acidity. Acidity, a measure of proton stability, plays a crucial role in determining the reactivity of molecules. By understanding the factors that influence proton acidity, we can predict and control the behavior of chemical systems.
This article delves into the concept of proton acidity, exploring the impact of electronegativity, resonance, and inductive effects on proton stability. We will also examine the structural features that modulate acidity, including the influence of alkyl groups, aromatic rings, and heteroatoms.
Furthermore, we will discuss the practical applications of proton acidity in organic chemistry reactions and biological systems.
Introduction
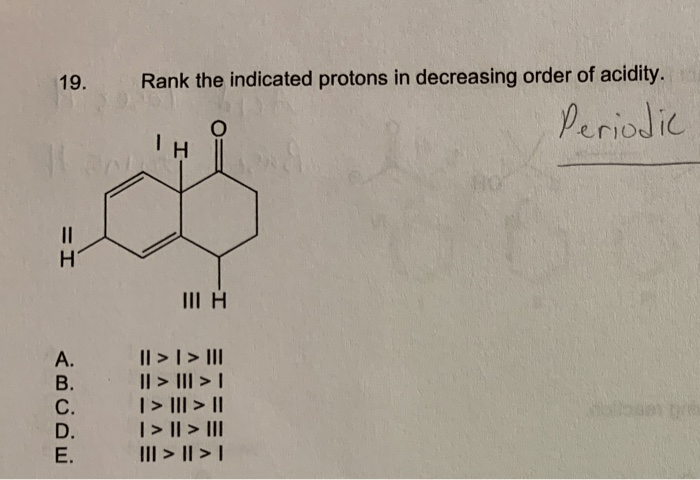
Acidity is a measure of the ability of a compound to donate a proton (H+). The more stable the conjugate base (the species formed when a proton is removed), the more acidic the compound. Proton acidity can be ranked in order of increasing acidity, with the most acidic protons being those that are most easily removed.
Factors Affecting Proton Acidity
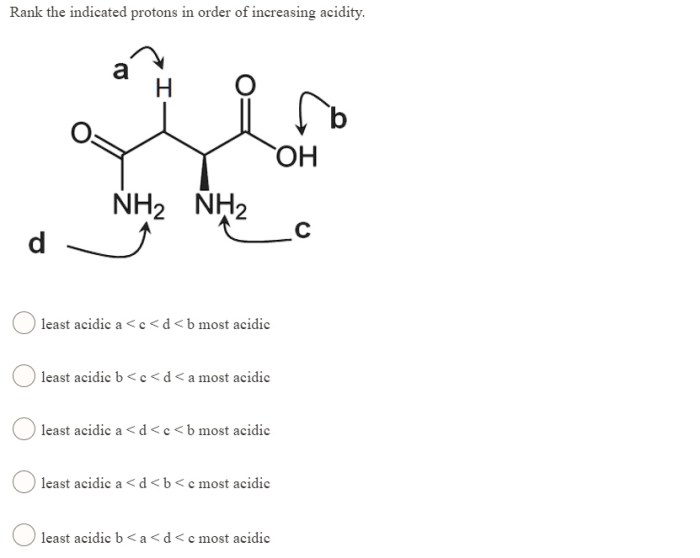
Electronegativity
Electronegativity is the ability of an atom to attract electrons towards itself. The more electronegative an atom, the more strongly it will hold onto its electrons, and the less likely it is to donate a proton. Therefore, compounds with more electronegative atoms tend to be less acidic.
Resonance
Resonance is a phenomenon that occurs when there are multiple possible Lewis structures for a molecule. Resonance can stabilize the conjugate base of a compound, making it more likely to donate a proton. Therefore, compounds with resonance structures tend to be more acidic.
Inductive Effects
Inductive effects are the effects that electron-withdrawing or electron-donating groups have on the acidity of a compound. Electron-withdrawing groups tend to decrease acidity, while electron-donating groups tend to increase acidity.
Structural Effects on Proton Acidity
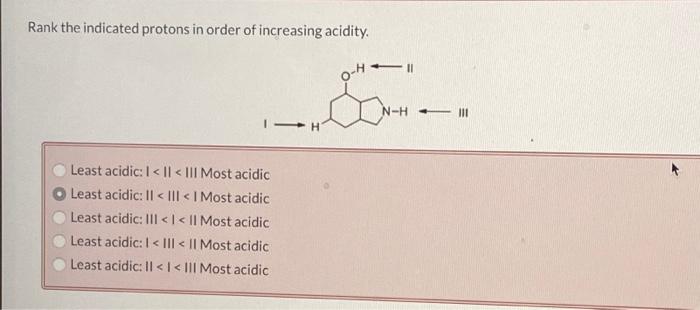
Alkyl Groups
Alkyl groups are electron-donating groups, which means they tend to increase the acidity of a compound. This is because alkyl groups push electrons towards the proton, making it more likely to be removed.
Aromatic Rings
Aromatic rings are electron-withdrawing groups, which means they tend to decrease the acidity of a compound. This is because aromatic rings pull electrons away from the proton, making it less likely to be removed.
Heteroatoms
Heteroatoms are atoms other than carbon and hydrogen. Heteroatoms can be either electron-withdrawing or electron-donating, depending on their electronegativity. Electron-withdrawing heteroatoms tend to decrease acidity, while electron-donating heteroatoms tend to increase acidity.
Applications of Proton Acidity: Rank The Indicated Protons In Order Of Increasing Acidity
Organic Chemistry Reactions, Rank the indicated protons in order of increasing acidity
Proton acidity is important in many organic chemistry reactions. For example, proton acidity is a factor in acid-base reactions, nucleophilic substitutions, and elimination reactions.
Biological Systems
Proton acidity is also important in biological systems. For example, proton acidity is a factor in enzyme catalysis and protein function.
Commonly Asked Questions
What is the relationship between proton acidity and proton stability?
Proton acidity is inversely proportional to proton stability. A more acidic proton is less stable and more likely to be released from its parent molecule.
How does electronegativity affect proton acidity?
Electronegative atoms withdraw electron density from the proton, making it more acidic. This is because the more electronegative the atom, the more it attracts the electrons in the covalent bond, leaving the proton with a higher positive charge.
What is the role of resonance in proton acidity?
Resonance stabilizes the negative charge that results from proton removal, making the proton more acidic. This is because resonance delocalizes the negative charge over multiple atoms, reducing the electron density on any one atom.